Calcium Hydroxide and Perchloric Acid Balanced Equation
Calcium hydroxide ironlll. 0000003271 00000 n Perchloric acid.

How To Balance Hclo4 Ca Oh 2 Ca Clo4 2 H2o Perchloric Acid Calcium Hydroxide Youtube
H₃Oaq OHaq 2H₂Ol.
. H2SO4 CaOH2 CaSo4 2H2O One molecule each of sulfuric acid and calcium hydroxide react to give one molecule of calcium sulfate and TWO molecules of water. The balanced chemical equation for hydroiodic acid and calcium hydroxide is as followsCa OH2 aq 2HI aq CaI2 aq 2H2O l Wiki User. Asked Jun 25 2017 in Chemistry by Lisas.
How to Balance HClO4 Ba OH2 Ba ClO42 H2O Perchloric acid Barium hydroxide Watch later. Phosphoric acid - diluted solution. A beaker of nitric acid is neutralized with calcium hydroxide.
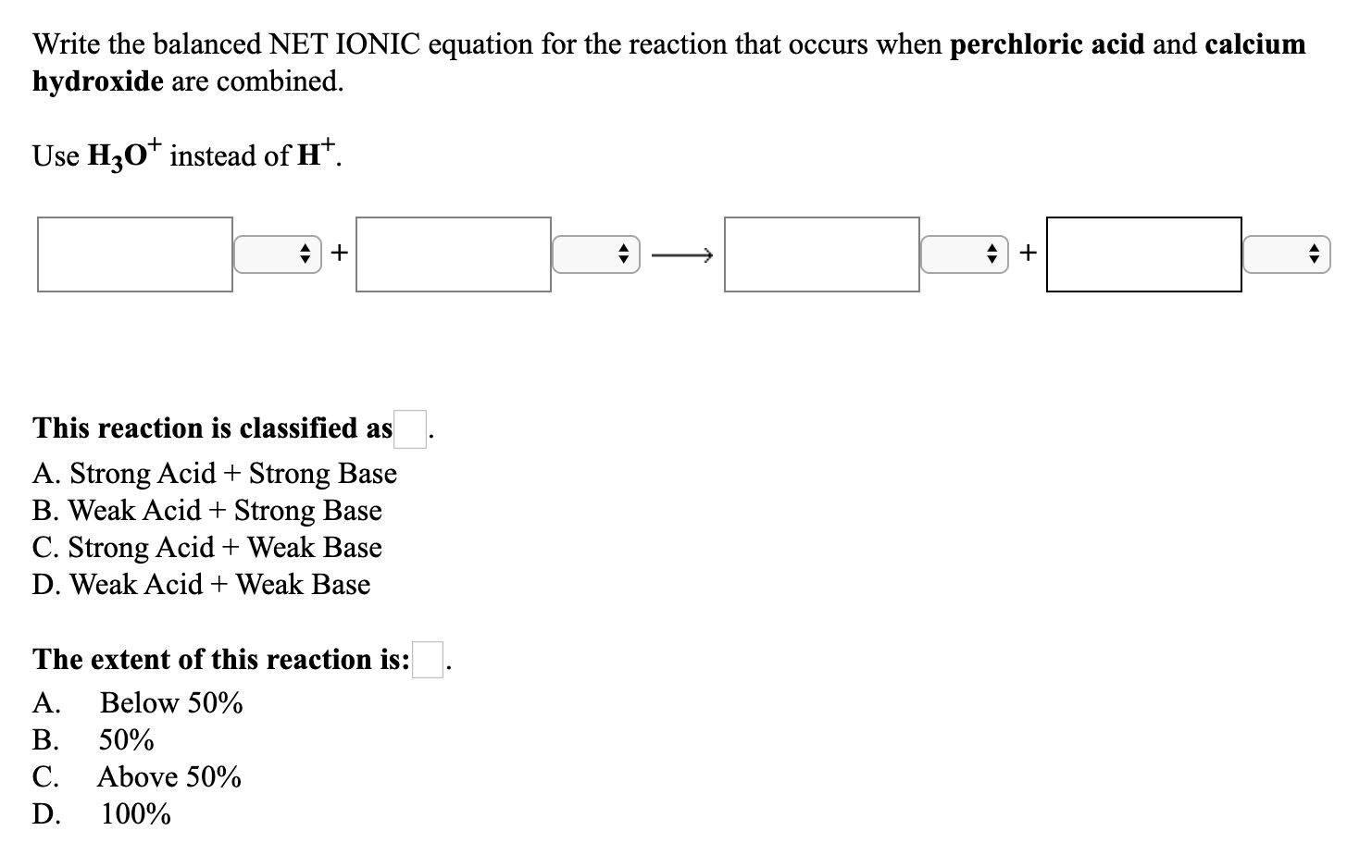
What is the net ionic equation for the reaction between a strong acid and a strong base. How to Balance HClO4 Ca OH2 Ca ClO42 H2O Perchloric acid Calcium hydroxide - YouTube. The balanced equation for ammonium hydroxide and perchloric acid is given belowNH4OH aq HCl aq --- NH4Cl aq H2O l Above is the balanced molecular Equation.
HClO4 Calcium hydroxide is a base and perchloric aci View the full answer Transcribed image text. HClO4 aq NaOH aq --- Na aq ClO4- aq H2O l This is a balanced chmical reaction since the products and reacts have a 11 ratio. Equation not just as H 2H2O Q1 driers containing a mixture of cobalt hydroxide No.
What is the correct formula for perchloric acid. CaClO42 H2O HClO4 CaOH2. The reaction of Perchloric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base.
Calcium hydroxide is added to perchloric acid. H2SO4 CaOH2 CaSo4 2H2O One molecule each of sulfuric acid and calcium hydroxide react to give one molecule of calcium sulfate and TWO molecules of water. Write the equation for the reaction associated with the Kb2 of carbonate CO32.
Mg OH 2 2HClO 4 Mg ClO 4 2 2H 2 O. Net Ionic Equation For Perchloric Acid And Potassium Hydroxide. Perchloric acid - concentrated cold solution.
HClO 4 aq KOH aq KClO 4 aq H 2 O. ClO 4- aq K aq KClO 4 aq D. Perchloric acid HCIO and potassium hydroxide KOH net ionic equation.
1 pts Question 7 Calcium hydroxide is added to perchloric acid. Lithium hydroxide and acetic acid 17. Portlandite Perchloric Acid Calcium Perchlorate Water.
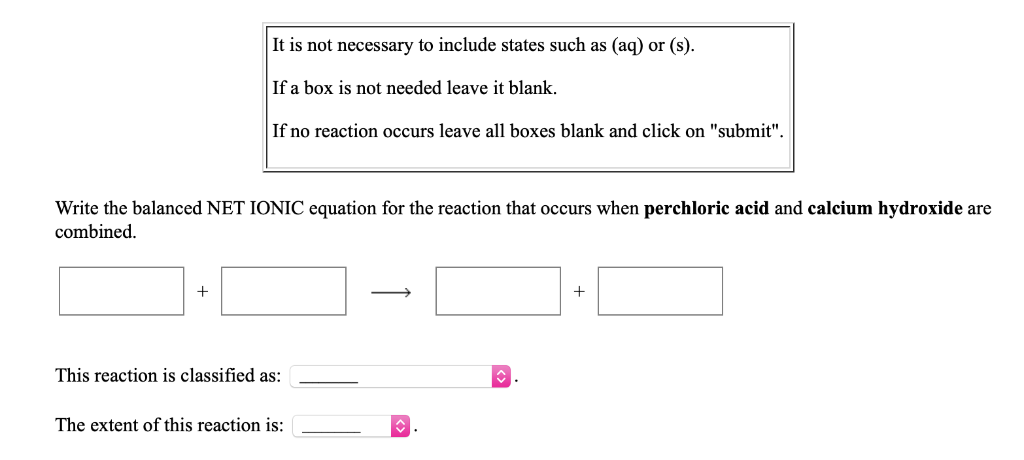
Please log in or register to answer this question. Write the balanced NET IONIC equation for the reaction that occurs when perchloric acid and calcium hydroxide are combined. The balanced equation will be.
Write the equation for the reaction associated with the Ka2 of sulfuric acid H2SO4. The balanced equation will be. CaClO42 H2O CaOH2 HClO4.
CaClO42 H2O CaClO2 H2O2. CaOH2 HClO4 CaClO2 H2O O. HClO 4 KOH KClO 4 H 2 O.
Check the balance Magnesium hydroxide react with perchloric acid to produce magnesium perchlorate and water. HClO 4 aq KOH aq KClO 4 s H 2 O. This answer is.
CaOH2 HClO4 H2O CaClO42. Write the balanced chemical equation for this reaction. Check the balance Perchloric acid react with potassium hydroxide to produce potassium perchlorate and water.
CaOH2 HClO4 CaClO2 O2 H2. Potassium hydroxide - saturated solution. 2H3Oaq CaOH2s.
Perchloric acid - diluted solution. Please log in or register to add a comment. CaOH2 HClO4 CaClO42 HOH CaOH2 HClO4 CaClO42 HOH.
Strong acids and strong bases are considered strong electrolytes and will dissociate completely. A Aqueous acetic acid is neutralized by aqueous potassium hydroxide. Write the balanced molecular and net ionic equations for each of the following neutralization reactions.
HClO2aqNaOHaq H2OlNaClO2aq net ionic equation. HClO 4 NaOH NaClO 4 H 2 O is a neutralization reaction also a double displacement. Calcium hydroxide acetic acid I cant figure out how to write the molecular and net ionic equation for the life of me.
CaOH2 b Perchloric acid. Calcium Hydroxide Perchloric Acid Calcium Perchlorate Water. Coefficient for perchloric acid.
This means that we will split them apart in the net ionic equation. A Calcium Hydroxide. Write a balanced molecular equation and a net ionic equation for his reaction.
3Ca OH 2 2H 3 PO 4 Ca 3 PO 4 2 6H 2 O. Perchloric acid with barium hydroxide. When a strong acid and a strong base are mixed they react according to the following net-ionic equation.
Check the balance Calcium hydroxide react with phosphoric acid to produce calcium orthophosphate and water. Write the balanced chemical equation for the reaction between hydrazoic acid and calcium hydroxide.
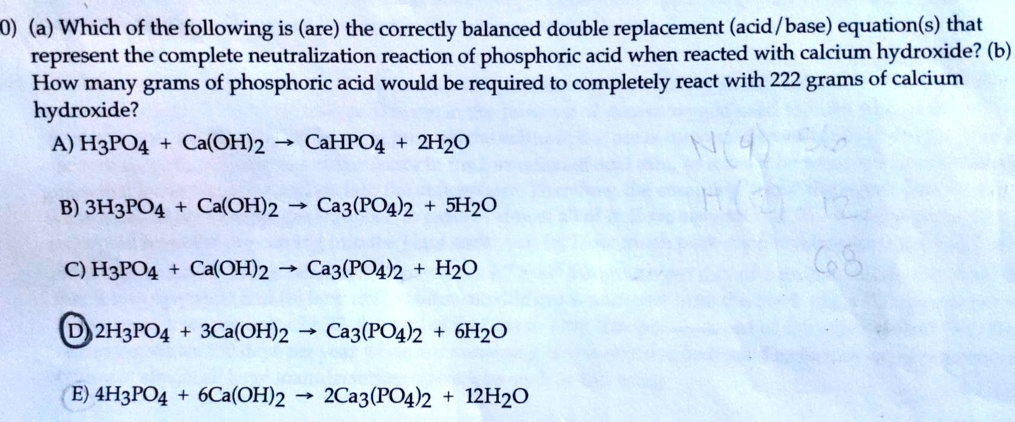
Solved 0 A Which Of The Following Is Are The Correctly Balanced Double Replacement Acid Base Equation S That Represent The Complete Neutralization Reaction Of Phosphoric Acid When Reacted With Calcium Hydroxide B How
How To Write The Net Ionic Equation For Hcl Ca Oh 2 Cacl2 H2o Youtube

Solved 12 How Many Moles Of Perchloric Acid Hcioa Are Chegg Com
Solved Write Balanced Ionic Equations For The Following Chegg Com

How To Balance Mg Oh 2 Hcl Mgcl2 H2o Magnesium Hydroxide Hydrochloric Acid Youtube

Solved Mg Oh 2 S B Mg3 Po4 2 S H3po4 Aq H20 1 Chegg Com
Solved Write The Balanced Net Ionic Equation For The Chegg Com

How To Balance Hclo4 Ca Oh 2 Ca Clo4 2 H2o Perchloric Acid Calcium Hydroxide Youtube
Solved A What Volume Of A 0 165 M Sodium Hydroxide Solution Chegg Com
How To Balance Hclo3 Ca Oh 2 Ca Clo3 2 H2o Youtube

Solved Write The Balanced Net Ionic Equation For The Chegg Com

Solved A Choose A Balanced Net Ionic Equation For The Chegg Com

How To Write The Net Ionic Equation For Hclo4 Ca Oh 2 Ca Clo4 2 H2o Youtube

How To Write The Net Ionic Equation For Hclo4 Ca Oh 2 Ca Clo4 2 H2o Youtube

Calcium Hydroxide Neutralizes Perchloric Acid What Is The Sum Of The Coefficients Of The Balanced Equation For This Reaction Study Com

Chapter 4 The Major Classes Of Chemical Reactions 4 1 The Role Of Water As A Solvent 4 2 Precipitation Reactions And Acid Base Reactions 4 3 Oxidation Ppt Download
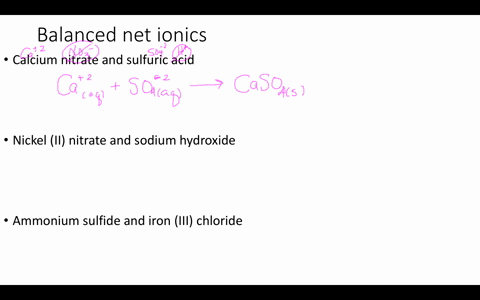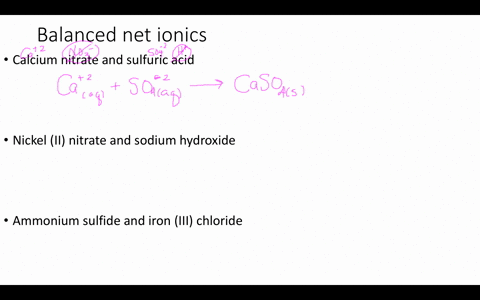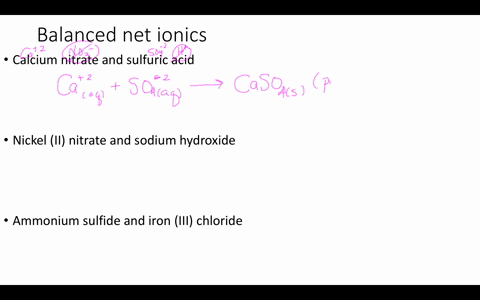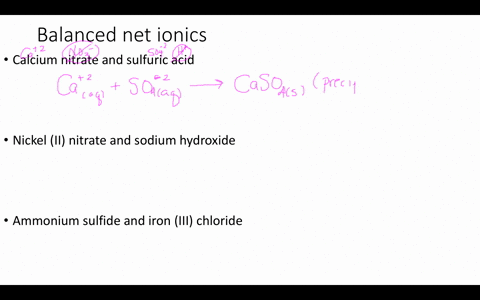
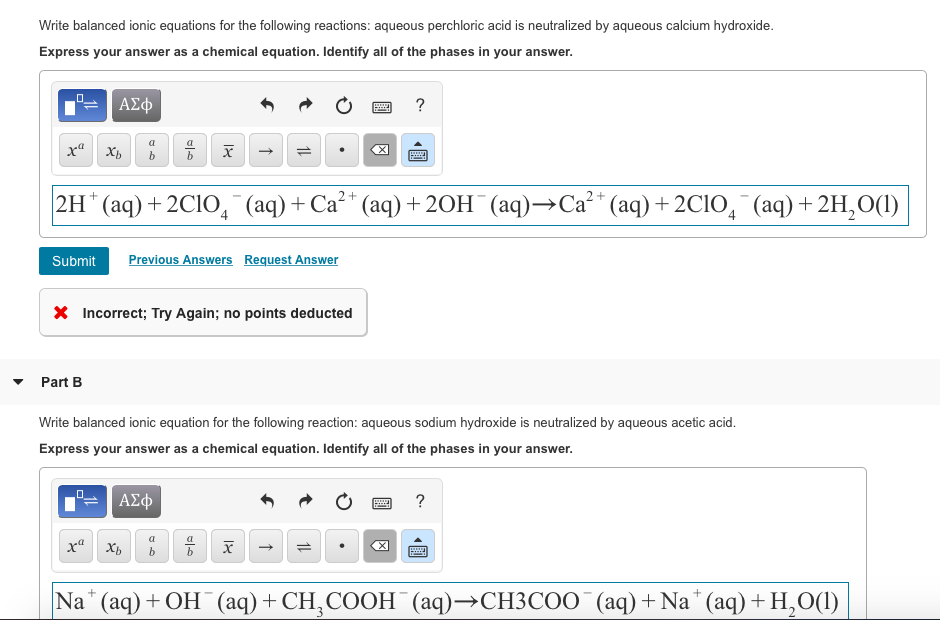
Solved Write Balanced Ionic Equations For The Following Reactions A Aqueous Perchloric Acid Is Neutralized By Aqueous Calcium Hydroxide B Aqueous Sodium Hydroxide Is Neutralized By Aqueous Acetic Acid

How To Write The Net Ionic Equation For Hcl Ca Oh 2 Cacl2 H2o Youtube

Solved Write Balanced Ionic Equations For The Following Reactions A Aqueous Perchloric Acid Is Neutralized By Aqueous Calcium Hydroxide B Aqueous Sodium Hydroxide Is Neutralized By Aqueous Acetic Acid